The kidneys, a pair of bean-shaped organs nestled beneath the rib cage, carry the weighty responsibility of maintaining the body’s internal homeostasis. They meticulously filter waste products, balance electrolytes, regulate blood pressure, and produce vital hormones. When this sophisticated machinery falters, the symptoms can often be vague, mirroring other conditions, or, alarmingly, non-existent until the disease has reached an advanced stage. Diagnostic blood and urine tests can certainly signal that something is amiss—elevated creatinine, proteinuria, or hematuria—but they frequently fail to deliver the definitive “why.” They are the red flags, not the map. In the face of ambiguity or the need to precisely stage a known disease, clinicians often turn to the procedural bedrock of nephrology: the kidney, or renal, biopsy. This procedure transitions diagnosis from mere biochemical inference to direct, cellular-level evidence.
They are the red flags, not the map.
A kidney biopsy is, fundamentally, a minimally invasive surgical procedure designed to procure a small cylinder of tissue—a “core”—from one of the kidneys for microscopic examination. The primary aim is to establish a clear, histological diagnosis. While imaging techniques like ultrasound or CT scans offer invaluable structural information—identifying masses, cysts, or blockages—they cannot reveal the underlying pathology at the cellular level. Is the patient suffering from minimal change disease, focal segmental glomerulosclerosis, or perhaps a rapidly progressing form of glomerulonephritis? Are immune complexes being deposited, and if so, what type? The answers to these intricate questions lie locked within the tissue architecture, requiring the biopsy to unlock them. This critical distinction between functional and structural pathology underscores the biopsy’s irreplaceable role in formulating a targeted, effective treatment strategy.
Indications for Direct Tissue Examination
The decision to perform a renal biopsy is never taken lightly, as it involves inherent, albeit low, risks. It is reserved for clinical scenarios where the potential benefit of obtaining a precise diagnosis and guiding therapy far outweighs the procedural risk. One of the most common indications is unexplained acute or rapidly progressive renal failure, particularly when post-renal causes (obstruction) and pre-renal causes (hypovolemia) have been ruled out. Here, identifying the specific cause—such as acute interstitial nephritis or vasculitis—is paramount for immediate, life-saving therapy.
The decision to perform a renal biopsy is never taken lightly, as it involves inherent, albeit low, risks.
Another major category for biopsy is persistent, significant proteinuria (protein in the urine) or hematuria (blood in the urine), especially when combined with deteriorating kidney function. The presence of nephrotic syndrome—characterized by massive proteinuria, hypoalbuminemia, and edema—almost always mandates a biopsy to differentiate between various forms of primary and secondary glomerular diseases, such as lupus nephritis or amyloidosis, which require vastly different immunosuppressive regimens. Furthermore, biopsies are crucial in monitoring the progression of known conditions, assessing the effectiveness of treatment protocols, and investigating the cause of allograft dysfunction—the deterioration of a transplanted kidney—where the tissue sample can distinguish between rejection and recurrent disease.
The Standard Percutaneous Approach
The vast majority of kidney biopsies are performed using the percutaneous approach, often referred to as a “closed” biopsy. This technique avoids a large surgical incision and is typically performed by a nephrologist or an interventional radiologist. The patient is positioned prone (face-down) on a table, and the procedure is usually conducted under conscious sedation and local anesthesia to numb the skin and deeper tissues at the biopsy site, typically on the back near the kidney. The cornerstone of the procedure is the real-time use of imaging, usually ultrasound, to precisely locate the lower pole of the kidney—the preferred site due to its minimal association with large blood vessels.
The patient is positioned prone (face-down) on a table, and the procedure is usually conducted under conscious sedation and local anesthesia.
Once the target area is identified, a small incision, often just a nick, is made. A specialized biopsy needle, often a spring-loaded, automated device, is then guided through the skin, muscle, and renal capsule and into the cortex of the kidney. The patient is usually instructed to hold their breath at the moment the tissue sample is taken, minimizing kidney movement. This ensures the needle is still and the core is collected cleanly. Typically, two to three cores are needed to ensure enough glomeruli (the filtering units) and tubulointerstitial tissue are obtained for comprehensive analysis across various laboratory techniques. The efficiency and low invasiveness of the percutaneous method make it the gold standard, offering a high diagnostic yield with a relatively short recovery time.
Tissue Processing and Microscopic Analysis
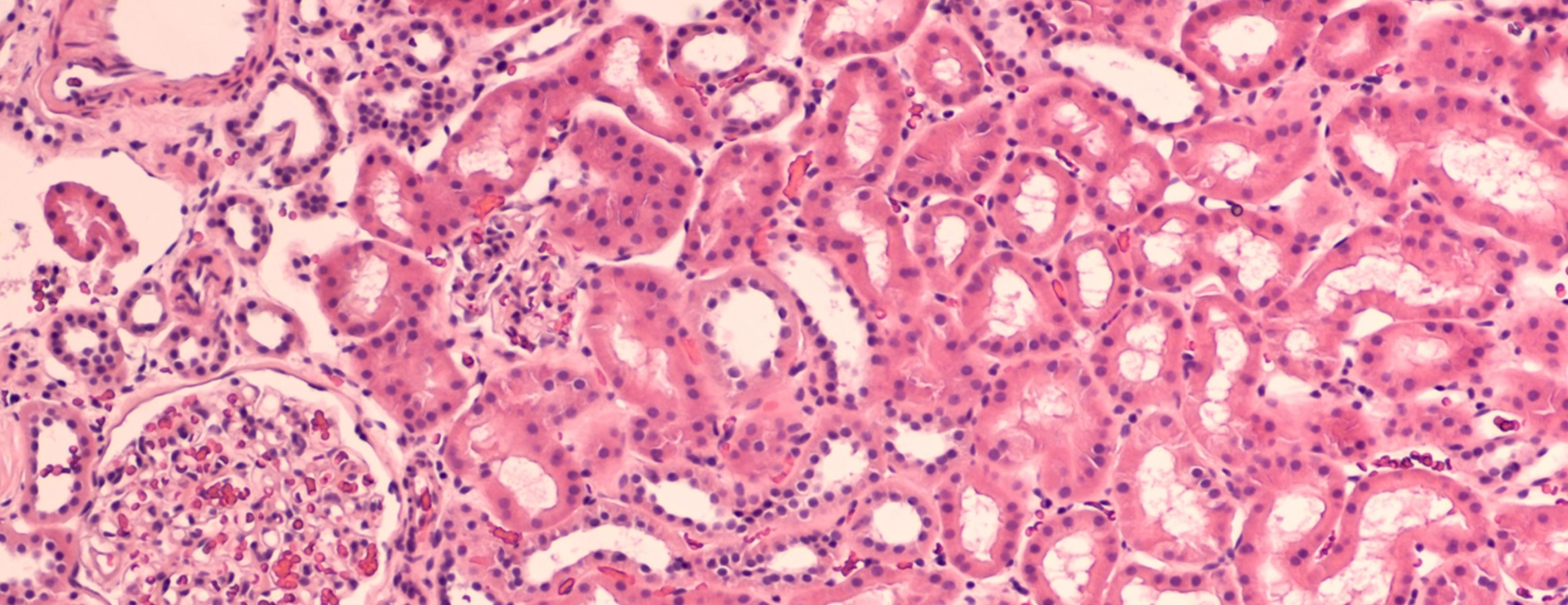
The true value of the kidney biopsy lies not in the collection of the tissue, but in the meticulous analysis that follows. The obtained tissue cores are immediately divided and processed using three complementary, non-negotiable techniques. The first, and most foundational, is light microscopy (LM), where the tissue is fixed, sectioned into thin slices, and stained with various dyes (like Hematoxylin and Eosin, Periodic Acid-Schiff) to reveal the general structure and cellular detail, helping to assess inflammation, fibrosis, and sclerosis.
The true value of the kidney biopsy lies not in the collection of the tissue, but in the meticulous analysis that follows.
The second technique is immunofluorescence (IF), which involves freezing a segment of the tissue and applying fluorescent antibodies designed to tag specific immune components, such as immunoglobulins (IgG, IgA, IgM), complement proteins (C3, C1q), and fibrin. The pattern and location of these deposits are crucial for diagnosing immune complex-mediated diseases, for example, the granular deposition of IgA in IgA nephropathy. The third component is electron microscopy (EM). This technique provides ultra-high magnification to visualize minute subcellular structures, like the podocytes, glomerular basement membrane, and endothelial cells, helping to identify subtle pathology, such as effacement of the podocyte foot processes seen in minimal change disease or dense deposit patterns. The final diagnosis synthesizes the findings from all three modalities, requiring specialized expertise from a renal pathologist.
Potential Risks and Mitigating Strategies
While a kidney biopsy is generally considered safe, it is an invasive procedure that carries certain, well-documented risks. The most frequent and significant complication is bleeding, as the kidneys are highly vascularized organs. Minor hematuria (blood in the urine) is common, but major bleeding requiring blood transfusion or intervention occurs in a small percentage of cases, typically less than $5\%$. A rare but more serious complication is a perirenal hematoma—a collection of blood around the kidney—which may necessitate a prolonged hospital stay.
The most frequent and significant complication is bleeding, as the kidneys are highly vascularized organs.
Mitigating these risks involves strict pre-procedural protocols. Patients must undergo a thorough assessment of their coagulation status, including platelet count and prothrombin time, and must temporarily discontinue any antiplatelet or anticoagulant medications (like aspirin or warfarin) several days before the procedure. Post-procedural management is equally critical: the patient is monitored closely for several hours (often $12$ to $24$ hours) in the hospital, typically required to lie flat to maintain pressure on the biopsy site and to allow for frequent vital sign checks. The use of ultrasound guidance has significantly reduced the risk profile compared to blind biopsies, by ensuring precise needle placement and avoidance of major vessels.
Contraindications and Procedural Alternatives
Not every patient is a candidate for a percutaneous kidney biopsy. Absolute contraindications are conditions that make the procedure excessively risky. These include an uncontrolled bleeding diathesis (severe clotting disorder), uncontrolled hypertension, a solitary functional kidney (excluding a transplanted kidney), and active pyelonephritis (kidney infection). Severe obesity or inability to cooperate and hold one’s breath during the procedure may also pose significant challenges.
Not every patient is a candidate for a percutaneous kidney biopsy.
In scenarios where a percutaneous biopsy is contraindicated or technically difficult, alternative methods may be employed. The transjugular kidney biopsy involves threading a catheter through the jugular vein in the neck down to the renal vein. A needle is then deployed through the wall of the vein into the kidney tissue. This technique is preferred for patients with severe coagulopathy or those who are extremely obese. Another, less common alternative is the open (surgical) biopsy, which requires a small surgical incision under general anesthesia to visually expose the kidney and obtain the tissue sample. While more invasive, it allows for direct visualization of the kidney and meticulous control of bleeding and is occasionally used when other methods have failed or are contraindicated.
Post-Biopsy Care and Recovery
Following the successful acquisition of the tissue sample, the period of post-biopsy care is crucial for minimizing complications and ensuring a smooth recovery. As noted, the patient is required to remain flat in bed for a period, typically $6$ to $12$ hours, to allow the small puncture site in the kidney to seal naturally. During this time, the nursing staff frequently checks the patient’s blood pressure, heart rate, and observes the urine for gross hematuria. Pain at the biopsy site is common but usually manageable with over-the-counter or prescribed analgesics.
The patient is required to remain flat in bed for a period, typically $6$ to $12$ hours, to allow the small puncture site in the kidney to seal naturally.
Upon discharge, patients are advised to restrict physical activity for at least one to two weeks, avoiding heavy lifting, strenuous exercise, and contact sports to prevent potential delayed bleeding. They are also instructed to watch for specific “red flag” symptoms that warrant immediate medical attention, such as large clots in the urine, severe pain that does not respond to medication, lightheadedness, or fever. Adherence to these post-procedural guidelines is instrumental in preventing the majority of late-onset complications, highlighting the patient’s role as a co-manager of the recovery process.
Interpreting the Biopsy Report
The final kidney biopsy report is often a complex, multi-page document that summarizes the findings from the light, immunofluorescence, and electron microscopy studies. It is typically structured to address the key components of the kidney: the glomeruli, the tubulointerstitium, and the blood vessels. The report will quantify the degree of damage—for example, the percentage of glomeruli that are globally sclerosed or the extent of interstitial fibrosis. The pathologist’s diagnosis will be precise, moving beyond broad categories like “nephritis” to specific entities, such as “C3 Glomerulonephritis” or “Class IV Lupus Nephritis.”
The final kidney biopsy report is often a complex, multi-page document that summarizes the findings.
Understanding the nuances of the report is essential for the treating nephrologist. The report often provides prognostic information, suggesting whether the disease is chronic and irreversible (indicated by extensive fibrosis) or still largely active and responsive to therapy (indicated by cellular crescent formation). The final diagnosis dictates the treatment plan, guiding the choice and intensity of immunosuppressive agents, such as corticosteroids, cyclophosphamide, or calcineurin inhibitors. A detailed discussion between the nephrologist and the patient is always necessary to translate the histological findings into a practical, personalized management plan.
Biopsy in the Transplant Setting
Kidney transplant recipients form a special cohort for whom the kidney biopsy is a frequently used and critical diagnostic tool. After transplantation, the kidney, or allograft, is constantly at risk of injury from various sources. If routine monitoring shows a rise in serum creatinine or new proteinuria, a biopsy is often performed promptly to determine the cause of the allograft dysfunction. The distinction between the two major causes—cellular or antibody-mediated rejection and recurrence of the original kidney disease—is clinically urgent.
Kidney transplant recipients form a special cohort for whom the kidney biopsy is a frequently used and critical diagnostic tool.
Rejection is an immune process targeting the foreign organ and requires immediate, aggressive escalation of immunosuppression. Recurrence of the primary disease, such as IgA nephropathy or focal segmental glomerulosclerosis, requires a different, tailored management approach. Biopsies are also used to stage the degree of chronic injury in the allograft, using standardized classification systems like the Banff classification, which helps predict long-term graft survival. The biopsy, in this setting, acts as the ultimate surveillance mechanism, allowing for the timely modification of immunosuppressive therapy to maximize the life of the transplanted organ.
The Evolution of Diagnostic Techniques
While the fundamental procedure of obtaining a tissue core has remained relatively consistent for decades, the surrounding diagnostic landscape is continually evolving. Traditional reliance on $2D$ tissue sections is increasingly being complemented by advanced molecular and genetic studies performed on the same biopsy sample. Techniques such as gene expression profiling and next-generation sequencing are beginning to provide deeper insights into the underlying mechanisms of disease, potentially identifying biomarkers that predict response to therapy or long-term prognosis.
The surrounding diagnostic landscape is continually evolving.
Furthermore, the integration of computational pathology and machine learning algorithms is enhancing the speed and consistency of biopsy interpretation. These tools can quantify cellular changes and immune deposits with a precision that aids the human pathologist, particularly in complex cases. The future may also involve less invasive sampling methods, such as liquid biopsies, which analyze cell-free DNA or exosomes in the urine or blood, though these are not yet a substitute for the structural detail provided by a tissue biopsy. Nonetheless, the core role of the renal biopsy—providing direct evidence of renal pathology—remains unchallenged as the ultimate diagnostic standard in nephrology.
